Item 4
Question 1
For parts of the free-response question that require calculations, clearly...

Chemistry, 02.12.2020 06:20 queennajas
Item 4
Question 1
For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate.
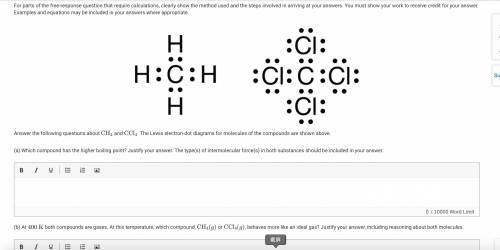
The figure presents two Lewis electron-dot diagrams. In the first diagram, a C atom is surrounded by 4 pairs of electrons, each of which is shared with an H atom. In the second diagram, a C atom is surrounded by 4 pairs of electrons, each of which is shared with a C l atom. Each C l atom has 3 additional pairs of nonbonding electrons.
Answer the following questions about CH4
and CCl4
. The Lewis electron-dot diagrams for molecules of the compounds are shown above.
(a) Which compound has the higher boiling point? Justify your answer. The type(s) of intermolecular force(s) in both substances should be included in your answer.
(b) At 400K
both compounds are gases. At this temperature, which compound, CH4(g)
or CCl4(g)
, behaves more like an ideal gas? Justify your answer, including reasoning about both molecules.
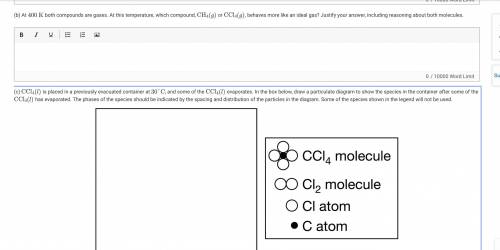
(c) CCl4(l)
is placed in a previously evacuated container at 30°C
, and some of the CCl4(l)
evaporates. In the box below, draw a particulate diagram to show the species in the container after some of the CCl4(l)
has evaporated. The phases of the species should be indicated by the spacing and distribution of the particles in the diagram. Some of the species shown in the legend will not be used.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1


You know the right answer?
Questions

History, 29.09.2019 23:50

Computers and Technology, 29.09.2019 23:50

Social Studies, 29.09.2019 23:50




Social Studies, 29.09.2019 23:50

Social Studies, 29.09.2019 23:50



Mathematics, 29.09.2019 23:50




Geography, 30.09.2019 00:00




Mathematics, 30.09.2019 00:00