Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
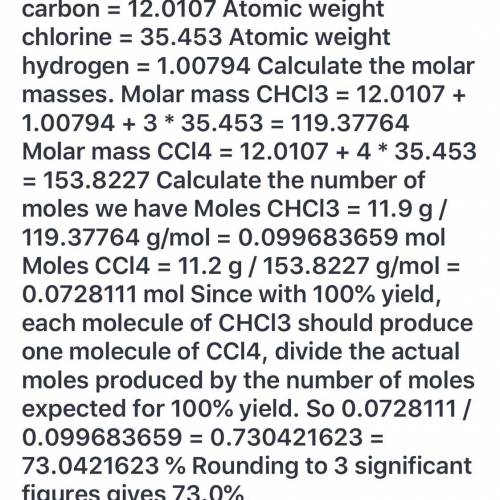
The reaction of 11.9 g of CHCl3 with excess chlorine produced 11 grams of CCl4, carbon tetrachloride...
Questions

Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01

Mathematics, 21.07.2020 21:01




Mathematics, 21.07.2020 21:01





Mathematics, 21.07.2020 21:01





Biology, 21.07.2020 21:01