
Chemistry, 01.12.2020 03:20 christianfielding336
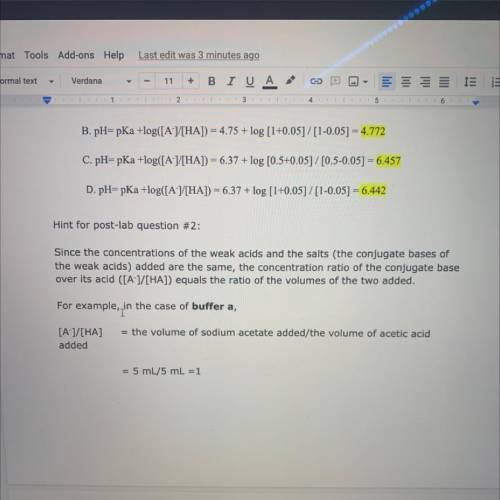
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjugate bases of
the weak acids) added are the same, the concentration ratio of the conjugate base
over its acid ([A-]/[HA]) equals the ratio of the volumes of the two added.
For example, in
in the case of buffer a,
= the volume of sodium acetate added/the volume of acetic acid
[A-]/[HA]
added
= 5 mL/5 mL =1

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1



Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Hint for post-lab question #2:
Since the concentrations of the weak acids and the salts (the conjug...
Questions






















