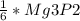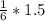Chemistry, 30.11.2020 18:20 descampbell2001
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess reactant that remains at the end of the reaction. Include math to justify your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess
reactant that re...
Questions

Mathematics, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01


Mathematics, 23.08.2020 01:01



Computers and Technology, 23.08.2020 01:01

Mathematics, 23.08.2020 01:01




Social Studies, 23.08.2020 01:01


Mathematics, 23.08.2020 01:01



Business, 23.08.2020 01:01

History, 23.08.2020 01:01

Social Studies, 23.08.2020 01:01