Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 14:00
Which statement describes the arrhenius interpretation of acids and bases?
Answers: 1
You know the right answer?
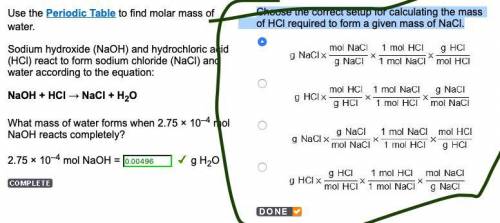
Choose the correct setup for calculating the mass of HCl required to form a given mass of NaCl.
Questions


Spanish, 29.10.2020 16:50






Computers and Technology, 29.10.2020 16:50



Biology, 29.10.2020 16:50