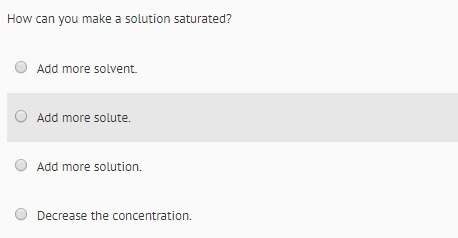
Chemistry, 29.11.2020 21:50 fqjenfiq6959
From the reaction C10H8(s) + 12O2(g) = 10CO2(g) + 4H2O(l) ΔrH° = –5153.0 kJ mol–1 and the enthalpies of formation of CO2 and H2O (–393.5 kJ mol–1 and –285.8 kJ mol–1, respectively) calculate the enthalpy of formation of naphthalene (C10H8).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
From the reaction C10H8(s) + 12O2(g) = 10CO2(g) + 4H2O(l) ΔrH° = –5153.0 kJ mol–1 and the enthalpies...
Questions


Business, 05.05.2020 11:40

Mathematics, 05.05.2020 11:40



Mathematics, 05.05.2020 11:41


Geography, 05.05.2020 11:41



Mathematics, 05.05.2020 11:41



Mathematics, 05.05.2020 11:41



History, 05.05.2020 11:41

Mathematics, 05.05.2020 11:41


Arts, 05.05.2020 11:41