

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
What is the molarity of a solution composed of 5.85 g of potassium iodide, KI, dissolved
in enough...
Questions


Mathematics, 03.05.2021 22:00

Mathematics, 03.05.2021 22:00


Mathematics, 03.05.2021 22:00

English, 03.05.2021 22:00



Biology, 03.05.2021 22:00

Mathematics, 03.05.2021 22:00

Mathematics, 03.05.2021 22:00






Mathematics, 03.05.2021 22:00

Mathematics, 03.05.2021 22:00


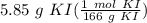 = 0.035241 mol KI
= 0.035241 mol KI

