
Chemistry, 29.11.2020 14:40 johndoesnutz4690
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of acetic acid is 1.76×10^–5 at 25°C. What is the pH of this mixture at 0°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of ace...
Questions

Mathematics, 01.07.2021 16:40




English, 01.07.2021 16:40

World Languages, 01.07.2021 16:40


Biology, 01.07.2021 16:40

Engineering, 01.07.2021 16:40



Computers and Technology, 01.07.2021 16:40

Social Studies, 01.07.2021 16:40

Mathematics, 01.07.2021 16:40

Mathematics, 01.07.2021 16:40

Mathematics, 01.07.2021 16:40



Advanced Placement (AP), 01.07.2021 16:40

Mathematics, 01.07.2021 16:40

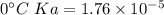
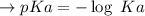
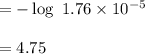
![pH = pKa + \log \frac{[sodium \ acetate]}{[acetic \ acid]}](/tpl/images/0932/3481/1f523.png)
![= 4.75 + \log \frac{[0.123]}{[0.291]}\\\\= 4.75+ \lg(0.422680412)\\\\=4.75-0.373987878\\\\=4.37601212\\\\=4.37](/tpl/images/0932/3481/5061d.png)


