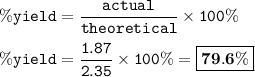Chemistry, 29.11.2020 08:50 zitterkoph
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
In this reaction, 1.87 g of indigo (C16H10N2O2) are produced.
If the theoretical yield is 2.35 g of C16H10N2O2, what is the percent yield of indigo?
Round to the nearest decimal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Can someone please help me with this?-- 18 pts!
2C10H11NO4 + 2H2O → C16H10N2O2 + 2C2H4O2 + 4H2O
Questions



Mathematics, 16.03.2020 17:51



English, 16.03.2020 17:51


Computers and Technology, 16.03.2020 17:51












Computers and Technology, 16.03.2020 17:52