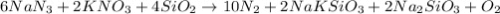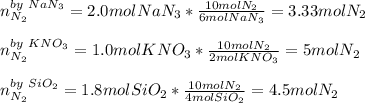Chemistry, 28.11.2020 03:20 isabellemaine
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide, releasing nitrogen gas and sodium potassium silicate (fine glass powder) 6 NaN3+ 2 KNO3 + 4 SiO210 N2+ 2 NaKSiO3+ 2 Na2SiO3 + 02 To conduct a similar reaction, 2.0 mol of NaN3, 1.0 mol of KNO3 and 1.8 mol of SiO2 are added. Which one is the limiting reactant? a) N2 b) O2 c) KNO3 d) SiO2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

You know the right answer?
When an airbag expands in a vehicle, sodium azide reacts with potassium nitrate and silicon dioxide,...
Questions

History, 19.04.2020 04:11

Physics, 19.04.2020 04:11

English, 19.04.2020 04:12


Mathematics, 19.04.2020 04:12


Mathematics, 19.04.2020 04:12









Mathematics, 19.04.2020 04:13

Advanced Placement (AP), 19.04.2020 04:13


Mathematics, 19.04.2020 04:13