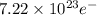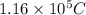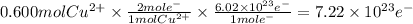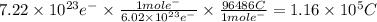Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 22:50
Compared with its corresponding unsaturated fatty acid, a saturated fatty acid has more hydrogen less hydrogen more oxygen less oxygen
Answers: 3

Chemistry, 23.06.2019 23:50
In the following reaction, oxygen is the excess reactant. sicl4 + o2 → sio2 + cl2 the table shows an experimental record for the above reaction. experimental record trial starting amount of sicl4 starting amount of o2 actual yield of sio2 1 120 g 240 g 38.2 g 2 75 g 50 g 25.2 g part 1: calculate the percentage yield for sio2 for trial 1. also, determine the leftover reactant for the trial. show your work. part 2: based on the percentage yield in trial 2, explain what ratio of reactants is more efficient for the given reaction.
Answers: 3


Chemistry, 24.06.2019 02:30
How many moles of k3po4 can be formed when 4.4 moles of h3po4 react with 3.8 moles of koh? h3po4 + koh yields h2o + k3po4be sure to balance the equation.1.3 mol k3po41.9 mol k3po42.2 mol k3po44.4 mol k3po4?
Answers: 3
You know the right answer?
You perform an electrochemical reaction in which 0.600 mol of Cu are reduced to solid Cu. How many c...
Questions



History, 24.06.2019 08:00

Computers and Technology, 24.06.2019 08:00



History, 24.06.2019 08:00


History, 24.06.2019 08:00


History, 24.06.2019 08:10






History, 24.06.2019 08:10