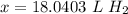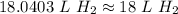Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

You know the right answer?
If 5.0 liters H2 (g) at STP is heated to a temperature of 985, pressure remaining constant, the new...
Questions

Geography, 21.10.2020 22:01






Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01




Mathematics, 21.10.2020 22:01






Mathematics, 21.10.2020 22:01

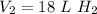
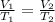
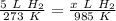 Cross-multiply:
Cross-multiply: 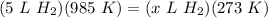 Multiply:
Multiply: 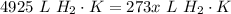 Isolate x:
Isolate x:  Rewrite:
Rewrite: