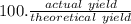Chemistry, 28.11.2020 01:00 augustxnicki
Under a certain set of conditions, the percent yield of a reaction that produces carbon dioxide is 75.0%. What mass in grams of CO2 will actually be recovered if the theoretical yield is 26.7 grams?


Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2


Chemistry, 23.06.2019 08:00
The biosphere of the earth is made up of . a. inorganic b. organic
Answers: 2
You know the right answer?
Under a certain set of conditions, the percent yield of a reaction that produces carbon dioxide is 7...
Questions


Mathematics, 02.04.2021 19:30


Computers and Technology, 02.04.2021 19:30

Mathematics, 02.04.2021 19:30

Mathematics, 02.04.2021 19:30





English, 02.04.2021 19:30

Computers and Technology, 02.04.2021 19:30


Mathematics, 02.04.2021 19:30




Mathematics, 02.04.2021 19:30


English, 02.04.2021 19:30

 %
% 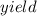 =
=