
Chemistry, 27.11.2020 14:00 lillysiege
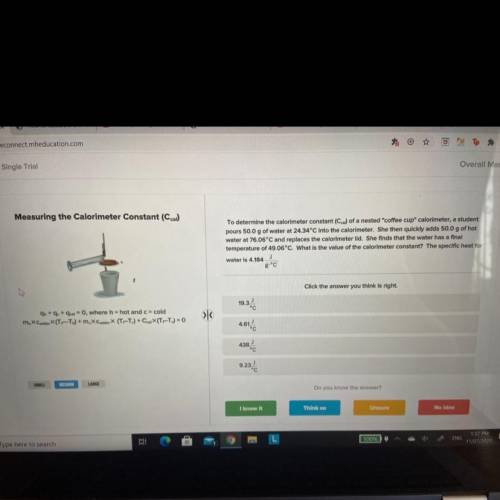
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours 50.0 g of water at 24.34°C into the calorimeter. She then quickly adds 50.0 g of hot
water at 76.06°C and replaces the calorimeter lid. She finds that the water has a final
temperature of 49.06°C. What is the value of the calorimeter constant? The specific heat for
J
water is 4.184

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours...
Questions


Mathematics, 17.05.2021 19:10



History, 17.05.2021 19:10


Mathematics, 17.05.2021 19:10


Mathematics, 17.05.2021 19:10

History, 17.05.2021 19:10

Spanish, 17.05.2021 19:10

History, 17.05.2021 19:10

Advanced Placement (AP), 17.05.2021 19:10


Mathematics, 17.05.2021 19:10

English, 17.05.2021 19:10

Physics, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10



