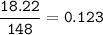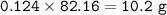Chemistry, 27.11.2020 08:20 blackdmnd7485
Adipic acid, C6H10O4, is a raw material for the making of nylon and it can be prepared in the laboratory by the following reaction between cyclohexene, C6H10, and sodium dichromate, Na2Cr2O7 in sulphuric acid.
3 C6H10 + 4 Na2Cr2O7 + 16 H2SO4 >
3 C6H10O4 + 4 Cr2(SO4)3 + 4Na2SO4 + 16 H2O
To prepare 12.5 grams of adipic acid in 68.6% yield requires how many grams of cyclohexen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Adipic acid, C6H10O4, is a raw material for the making of nylon and it can be prepared in the labora...
Questions

Mathematics, 15.12.2020 02:50

Mathematics, 15.12.2020 02:50

Mathematics, 15.12.2020 02:50

Social Studies, 15.12.2020 02:50

World Languages, 15.12.2020 02:50


Mathematics, 15.12.2020 02:50

Business, 15.12.2020 02:50

History, 15.12.2020 02:50


SAT, 15.12.2020 02:50

Mathematics, 15.12.2020 02:50



English, 15.12.2020 02:50

Mathematics, 15.12.2020 02:50



Mathematics, 15.12.2020 02:50

Spanish, 15.12.2020 02:50