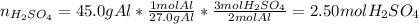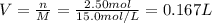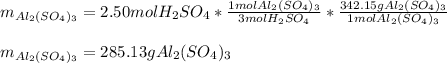Chemistry, 26.11.2020 21:50 JesusisLord2881
2Al(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g)a. Determine the volume (mL) of 15.0 M sulfuric acid needed to react with 45.0 g of aluminum to produce aluminum sulfate. b. Determine the % yield if 112 g of aluminum sulfate is produced under the above conditions.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
2Al(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g)a. Determine the volume (mL) of 15.0 M sulfuric acid nee...
Questions

Mathematics, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10


History, 29.04.2021 19:10



History, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10

Mathematics, 29.04.2021 19:10



Mathematics, 29.04.2021 19:10





Biology, 29.04.2021 19:10