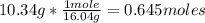Chemistry, 26.11.2020 19:20 madelynlittle5399
The temperature of a sample of CH4 gas (10.34 g) in a 50.0 L vessel at 1.33 atm is °C.
a.
984
b.
-195
c.
-1260
d.
-195

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

You know the right answer?
The temperature of a sample of CH4 gas (10.34 g) in a 50.0 L vessel at 1.33 atm is °C.
a.
Questions

English, 13.10.2019 05:20

Mathematics, 13.10.2019 05:20


Mathematics, 13.10.2019 05:20





Mathematics, 13.10.2019 05:20


Mathematics, 13.10.2019 05:20


Biology, 13.10.2019 05:20

Mathematics, 13.10.2019 05:20



Mathematics, 13.10.2019 05:20

Social Studies, 13.10.2019 05:20


Mathematics, 13.10.2019 05:20

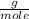 , that is, the mass present in one mole of an element or compound, the number of moles that 10.34 grams contains is calculated as:
, that is, the mass present in one mole of an element or compound, the number of moles that 10.34 grams contains is calculated as: