Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
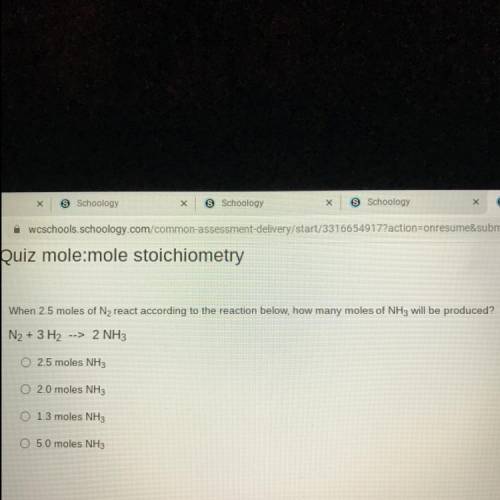
When 2.5 moles of N2 react according to the reaction below, how many moles of NH3 will be produced?...
Questions

Mathematics, 18.10.2019 06:30

Mathematics, 18.10.2019 06:30

Health, 18.10.2019 06:30

Social Studies, 18.10.2019 06:30




Health, 18.10.2019 06:30

History, 18.10.2019 06:30

English, 18.10.2019 06:30

Mathematics, 18.10.2019 06:30






Chemistry, 18.10.2019 06:30