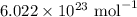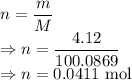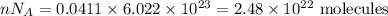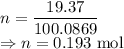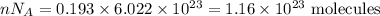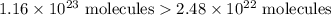I have 2 samples of solid chalk (aka calcium carbonate). Sample A has a total mass of 4.12 g and Sample B has a total mass of 19.37 g. What is the difference between the samples?
A) Sample B has more calcium carbonate molecules
B) Sample B has a larger ratio of carbon, oxygen, and calcium atoms
C) Sample B has more calcium ion than carbonate ions
D) Sample B must have some impurity

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
I have 2 samples of solid chalk (aka calcium carbonate). Sample A has a total mass of 4.12 g and Sam...
Questions


Chemistry, 16.04.2021 20:10








Mathematics, 16.04.2021 20:10


Chemistry, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10




History, 16.04.2021 20:10

Geography, 16.04.2021 20:10


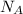 = Avogadro's number =
= Avogadro's number =