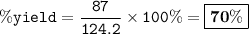Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
W...

Chemistry, 26.11.2020 01:00 pinkpearl20
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

You know the right answer?
Questions

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Health, 12.02.2021 07:40

Social Studies, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40

Mathematics, 12.02.2021 07:40







History, 12.02.2021 07:40



Health, 12.02.2021 07:40



Mathematics, 12.02.2021 07:40