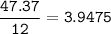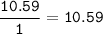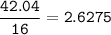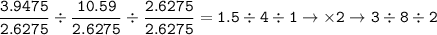Determine the empirical formula of a compound containing 47 37 grams of carbongrams of hydrogen, and of oxygen In an expenmen the me compound was determined to de 228 276 gis the molecular of the compound? For both questions your work or explain how you detemined the formulas by giving specille values used in calculations

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 11:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 300j of energy to the room?unit:
Answers: 1
You know the right answer?
Determine the empirical formula of a compound containing 47 37 grams of carbongrams of hydrogen, and...
Questions


Mathematics, 04.09.2019 05:30

Computers and Technology, 04.09.2019 05:30

Computers and Technology, 04.09.2019 05:30



Mathematics, 04.09.2019 05:30