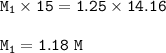Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
You complete titration of 15.0ml of unknown acid with 14.16ml of 1.25 M NaOH. What’s the molar it’s...
Questions



Chemistry, 31.10.2021 04:00





Biology, 31.10.2021 04:00



Mathematics, 31.10.2021 04:00

English, 31.10.2021 04:00

German, 31.10.2021 04:00






Mathematics, 31.10.2021 04:00