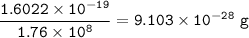Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
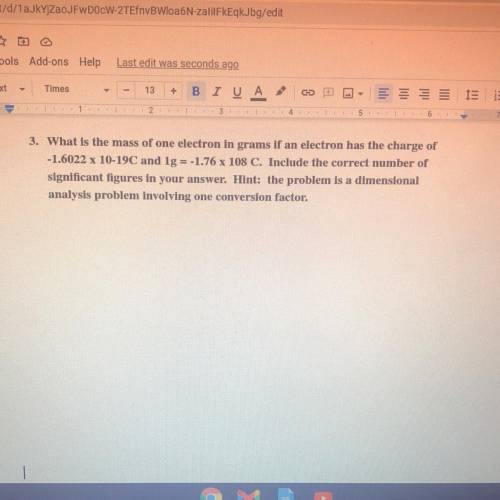
What is the mass of one electron in grams if an electron has the charge of
-1.6022 x 10-19C and lg...
Questions



Mathematics, 15.04.2020 17:44


History, 15.04.2020 17:44

Chemistry, 15.04.2020 17:44

Mathematics, 15.04.2020 17:44



Physics, 15.04.2020 17:44

Geography, 15.04.2020 17:44

Business, 15.04.2020 17:44


History, 15.04.2020 17:44





English, 15.04.2020 17:44

Computers and Technology, 15.04.2020 17:44