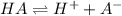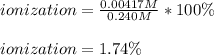Chemistry, 25.11.2020 03:30 nscarlisleh13
At equilibrium, the value of [H ] in a 0.240M solution of an unknown acid is 0.00417M . Determine the degree of ionization and the Ka of this acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
At equilibrium, the value of [H ] in a 0.240M solution of an unknown acid is 0.00417M . Determine th...
Questions

Mathematics, 03.09.2020 21:01


Biology, 03.09.2020 21:01




History, 03.09.2020 21:01


Mathematics, 03.09.2020 21:01

Mathematics, 03.09.2020 21:01

Computers and Technology, 03.09.2020 21:01

Advanced Placement (AP), 03.09.2020 21:01


Mathematics, 03.09.2020 21:01






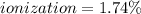
![ionization=\frac{[H^+]}{[HA]} *100\%](/tpl/images/0927/7847/7ad3f.png)