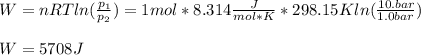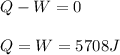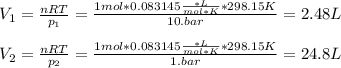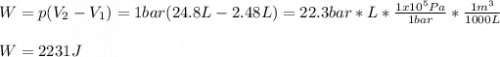Chemistry, 25.11.2020 02:30 twistedgamerhd12
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K.
Required:
a. Calculate the values of w, q, âU and âH?
b. Calculate w if the gas were to have expanded to the same final state against a constant pressure of 1 bar.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K....
Questions

Mathematics, 14.07.2019 07:30



Social Studies, 14.07.2019 07:30

History, 14.07.2019 07:30

Mathematics, 14.07.2019 07:30






Biology, 14.07.2019 07:30