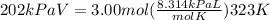Chemistry, 24.11.2020 18:10 jossfajardo50
What volume is occupied by 3.00 moles of nitrogen under a pressure of 202 kPa and a temperature of 323K? R = 8.314 kPa L/mol K INICI

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
What volume is occupied by 3.00 moles of nitrogen under a pressure of 202 kPa and a temperature of 3...
Questions





History, 31.01.2020 01:58

Mathematics, 31.01.2020 01:58

Health, 31.01.2020 01:58

Mathematics, 31.01.2020 01:58

Mathematics, 31.01.2020 01:58

History, 31.01.2020 01:58

Mathematics, 31.01.2020 01:58


English, 31.01.2020 01:58

Social Studies, 31.01.2020 01:58

Mathematics, 31.01.2020 01:58



Social Studies, 31.01.2020 01:58

Chemistry, 31.01.2020 01:58