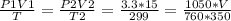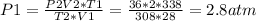2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If I raise the temperature to 350 K and lower the pressure to 1050 mmHg, what is the
new volume of the gas?
3) A gas that has a volume of 28 liters, a temperature of 65 °C, and an unknown pressure
has its volume increased to 36 liters and its temperature decreased to 35 °C. If I measure the
pressure after the change to be 2.0 atm, what was the original pressure of the gas?
work too pls

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If...
Questions


Mathematics, 21.01.2021 08:10



Mathematics, 21.01.2021 08:20

Advanced Placement (AP), 21.01.2021 08:20

Biology, 21.01.2021 08:20

English, 21.01.2021 08:20

Chemistry, 21.01.2021 08:20


Mathematics, 21.01.2021 08:20


Geography, 21.01.2021 08:20







Mathematics, 21.01.2021 08:20