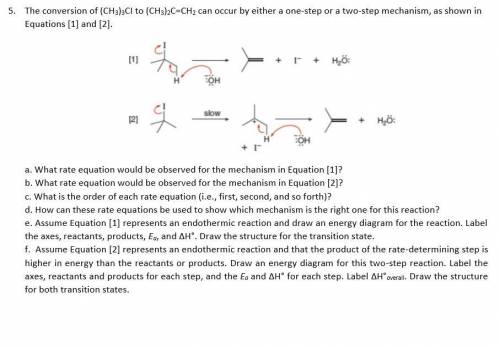
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as shown in
Equations (1) and (2]
[1]
있
+ I + H₂O
HK
OH
[2]
07
Slow
+ H₂O
+ I
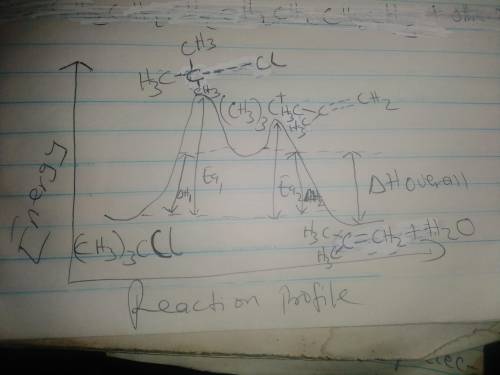
f) Assume Equation [a] represents an endothermic reaction
and that the product of the rate determining step is
higher in energy than the reactants or products.
Draw an energy dagram for this two-step reaction.
Label the axes, reactants and products for each step,
and the Ea and 4to for each step. Label 44° overall.
Draw the structure for both transition states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as sh...
Questions

Mathematics, 21.04.2020 17:31


Chemistry, 21.04.2020 17:31

History, 21.04.2020 17:31

History, 21.04.2020 17:31

Computers and Technology, 21.04.2020 17:31

Mathematics, 21.04.2020 17:31

Mathematics, 21.04.2020 17:31

Biology, 21.04.2020 17:31






Mathematics, 21.04.2020 17:31