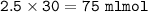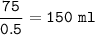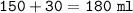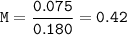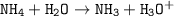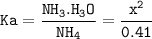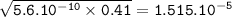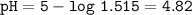Chemistry, 23.11.2020 08:50 raynamg2718
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of NH3 = 1.8 X 10-5
Write the balanced equation for this reaction.
What volume of 0.500 M HCl solution was added?
What is the molarity of the salt produced from the neutralization reaction?
What is the pH of the solution at the equivalence point?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of N...
Questions

History, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31


Biology, 25.11.2019 06:31





Mathematics, 25.11.2019 06:31

Mathematics, 25.11.2019 06:31



Mathematics, 25.11.2019 06:31


Mathematics, 25.11.2019 06:31


Physics, 25.11.2019 06:31