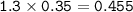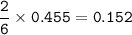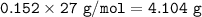What mass of aluminum (in g) would be
required to completely react with 1.30 L of
0.350 M HCl...

Chemistry, 23.11.2020 08:40 amadileaks
What mass of aluminum (in g) would be
required to completely react with 1.30 L of
0.350 M HCl in the following chemical
reaction?
2 Al(s) + 6 HCl(aq) → 2 AICI: (aq) + 3 H2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Questions



Mathematics, 24.11.2019 13:31

English, 24.11.2019 13:31









Mathematics, 24.11.2019 13:31




Geography, 24.11.2019 13:31


Biology, 24.11.2019 13:31