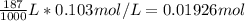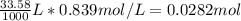Chemistry, 23.11.2020 08:10 eddrekas8564
Consider the following reaction. MgCl2(aq)+2NaOH(aq)⟶Mg(OH)2(s)+2NaC l(aq) A 187.0 mL solution of 0.103 M MgCl2 reacts with a 33.58 mL solution of 0.839 M NaOH to produce Mg(OH)2 and NaCl. Identify the limiting reactant.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
Consider the following reaction. MgCl2(aq)+2NaOH(aq)⟶Mg(OH)2(s)+2NaC l(aq) A 187.0 mL solution of 0....
Questions

Business, 05.05.2020 13:24

English, 05.05.2020 13:24



Social Studies, 05.05.2020 13:24

Chemistry, 05.05.2020 13:24

Mathematics, 05.05.2020 13:24


Business, 05.05.2020 13:24

Biology, 05.05.2020 13:24

English, 05.05.2020 13:24


Chemistry, 05.05.2020 13:24

Mathematics, 05.05.2020 13:24

English, 05.05.2020 13:24



English, 05.05.2020 13:24