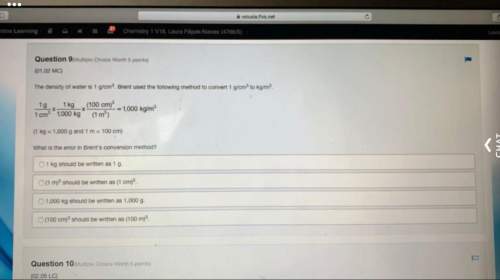
Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
What is the value of delta Hrxn for this equation:
NH3(g)+2O2(g)⟶HNO3(g)+H2O(g)
Based...
Based...
Questions

Mathematics, 02.10.2019 22:30







Mathematics, 02.10.2019 22:30



Biology, 02.10.2019 22:30


Mathematics, 02.10.2019 22:30


History, 02.10.2019 22:30





Chemistry, 02.10.2019 22:30

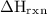 of the reaction can be 179 kJ/mol.
of the reaction can be 179 kJ/mol. 390
390