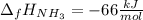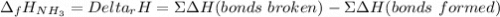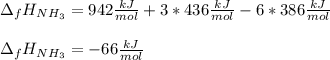Chemistry, 23.11.2020 02:30 afitzgerald
The formation of ammonia is represented by the equation N2(g) + 3H2(g) ⇌ 2NH3(g). Determine the enthalpy of formation of ammonia given the following mean bond enthalpies (kJmol-1): N≡N 942; H-H 436; N-H 386

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
The formation of ammonia is represented by the equation N2(g) + 3H2(g) ⇌ 2NH3(g). Determine the enth...
Questions

Computers and Technology, 19.11.2020 09:20

Arts, 19.11.2020 09:20


Physics, 19.11.2020 09:20

Physics, 19.11.2020 09:20

English, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20

Advanced Placement (AP), 19.11.2020 09:20

Biology, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20


English, 19.11.2020 09:20

Mathematics, 19.11.2020 09:20


Health, 19.11.2020 09:20

SAT, 19.11.2020 09:20


Mathematics, 19.11.2020 09:20