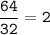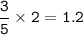Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Given the unbalanced combustion equation: C3H8 + O2 → CO2 + H2O, how many moles of carbon dioxide ar...
Questions

Mathematics, 17.06.2020 02:57

Mathematics, 17.06.2020 02:57


English, 17.06.2020 02:57



Mathematics, 17.06.2020 02:57

Biology, 17.06.2020 02:57









Mathematics, 17.06.2020 02:57


Chemistry, 17.06.2020 02:57