
Chemistry, 20.11.2020 21:50 estefaniapenalo
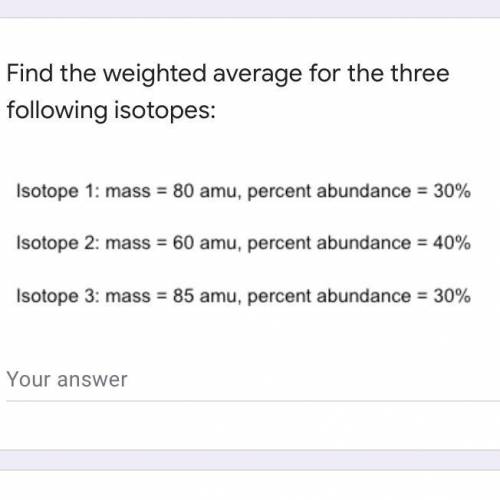
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abundance dance=30\% Isotope 2: mass = 60 amu, percent 1dance=40\% Isotope 3: mass = 85 amu, percent abundance 1dance=30\%

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abunda...
Questions


History, 22.03.2021 22:00


Mathematics, 22.03.2021 22:00



Mathematics, 22.03.2021 22:00

Chemistry, 22.03.2021 22:00


Mathematics, 22.03.2021 22:00



English, 22.03.2021 22:00

Social Studies, 22.03.2021 22:00

Mathematics, 22.03.2021 22:00


Advanced Placement (AP), 22.03.2021 22:00



Mathematics, 22.03.2021 22:00



