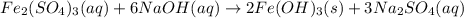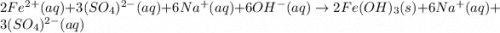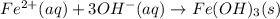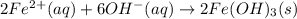Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
An aqueous solution of Iron(III) sulfate, Fe2(SO4)3, is mixed with an aqueous solution of Sodium hyd...
Questions


Engineering, 05.10.2019 14:30

Engineering, 05.10.2019 14:30


Engineering, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Computers and Technology, 05.10.2019 14:30

Mathematics, 05.10.2019 14:30