
Chemistry, 20.11.2020 17:10 quincyjosiah07
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molecular, medido a TPE, reacciona con un exceso de cloro molecular gaseoso. MHCl = 36.5 g/mol H2(g) + Cl2(g) → 2HCl(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molec...
Questions

Health, 02.09.2019 12:50

Mathematics, 02.09.2019 12:50



Biology, 02.09.2019 12:50

Biology, 02.09.2019 12:50

English, 02.09.2019 12:50


History, 02.09.2019 12:50

Computers and Technology, 02.09.2019 12:50

Physics, 02.09.2019 12:50

History, 02.09.2019 12:50





History, 02.09.2019 12:50



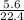 = 0.25mole
= 0.25mole 

