Chemistry, 20.11.2020 17:00 queenpaige2015
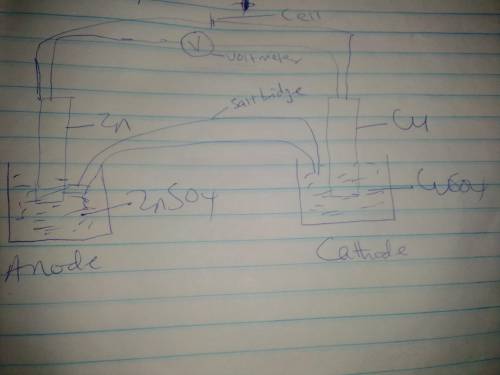
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76 V Cu^2+ + 2 e^> Cu E°cell = 0.34 V 1. Predict the standard potential of the cell at 298 K. 2. What is the minimum voltage that should be applied to the standard electrolytic cell found in question to cause zn2+ to be reduced to Zn?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76...
Questions







Computers and Technology, 08.12.2020 14:00



Computers and Technology, 08.12.2020 14:00

Physics, 08.12.2020 14:00




Mathematics, 08.12.2020 14:00

Mathematics, 08.12.2020 14:00



Chemistry, 08.12.2020 14:00