
Chemistry, 20.11.2020 14:00 biancaceja755
Methane (CH4) is the main component of marsh gas. Heating methane in the presence of sulfur produces carbon disulfide and hydrogen sulfide as the only products. (a) Write the balanced chemical equation for the reaction of methane and sulfur. (Use the lowest possible coefficients. Include states of matter under SATP conditions in your answer.)(b) Calculate the theoretical yield of carbon disulfide when g of methane is reacted with an equal mass of sulfur.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Methane (CH4) is the main component of marsh gas. Heating methane in the presence of sulfur produces...
Questions

Mathematics, 06.09.2019 02:10

Mathematics, 06.09.2019 02:10


Computers and Technology, 06.09.2019 02:10



Mathematics, 06.09.2019 02:10













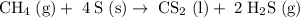
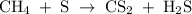
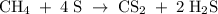
 molecular weight
molecular weight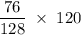 grams Carbon disulfide
grams Carbon disulfide

