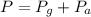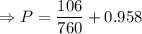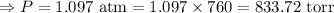Chemistry, 20.11.2020 14:00 rairaibabycakez9578
The mercury level in the open arm of an open-end manometer is 106 mm above the level in the arm connected to a tank of gas. If the barometric pressure is 0.958 atm, what is the pressure (in torr) of the gas in the tank?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1



Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
The mercury level in the open arm of an open-end manometer is 106 mm above the level in the arm conn...
Questions


History, 06.05.2020 21:08


Mathematics, 06.05.2020 21:08

History, 06.05.2020 21:08








History, 06.05.2020 21:08


Mathematics, 06.05.2020 21:08





English, 06.05.2020 21:08

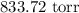
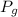 = Pressure of gas =
= Pressure of gas = 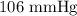
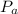 = Atmospheric pressure = 0.958 atm
= Atmospheric pressure = 0.958 atm