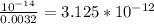Chemistry, 20.11.2020 14:00 sunshine52577oyeor9
Calculate the pH of the resulting solution if 32.0 mL of 0.320 M HCl(aq) is added to (a) 42.0 mL of 0.320 M NaOH(aq). (b) 22.0 mL of 0.420 M NaOH(aq).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
1) in saturated limewater, [h+ ]=3.98x10-13 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 2) in butter, [h+ ]=6.0x10-7 m. a) find [oh]-/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 3) in peaches, [oh]=3.16x10-11 m a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? / 4) during the course of the day, human saliva varies between being acidic and basic. if [oh]=3.16x10-8 m, a) find [h+ ]/ b) what is the ph? / c) is the solution acidic, basic, or neutral? /
Answers: 3

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Calculate the pH of the resulting solution if 32.0 mL of 0.320 M HCl(aq) is added to (a) 42.0 mL of...
Questions





Chemistry, 15.01.2022 08:20


Computers and Technology, 15.01.2022 08:20


Health, 15.01.2022 08:30

Mathematics, 15.01.2022 08:30



Mathematics, 15.01.2022 08:30


Mathematics, 15.01.2022 08:30


Biology, 15.01.2022 08:30


Mathematics, 15.01.2022 08:30

Mathematics, 15.01.2022 08:30