
Chemistry, 20.11.2020 14:00 eboniwiley
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. grams of water at 25.0 celsius. when the system comes to equilibrium the temperature of the system is 35.0 celsius. what is the mass in g of the iron? assume no heat is lost to the surroundingsSpecific heat/J. g^-1 K^-1iron 0.450water 4.184 (A) 4.48 g (B) 41.7 g (C) 387 g (D) 612 g

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. gra...
Questions

Mathematics, 29.01.2020 06:57




History, 29.01.2020 06:57

Mathematics, 29.01.2020 06:57



English, 29.01.2020 06:57

Chemistry, 29.01.2020 06:57

History, 29.01.2020 06:57

Mathematics, 29.01.2020 06:57

Mathematics, 29.01.2020 06:57



Biology, 29.01.2020 06:57

Computers and Technology, 29.01.2020 06:57


Mathematics, 29.01.2020 06:57

Biology, 29.01.2020 06:57

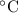 × (308 K - 298 K)
× (308 K - 298 K)

