Look at the balanced equation below:
C2H6O + 3 O2 → 2 CO2 + 3 H2O
If you produced 11.5...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Questions




Mathematics, 01.08.2019 01:20

English, 01.08.2019 01:20


Mathematics, 01.08.2019 01:20


Mathematics, 01.08.2019 01:20


Mathematics, 01.08.2019 01:20

History, 01.08.2019 01:20

Mathematics, 01.08.2019 01:20


Mathematics, 01.08.2019 01:20





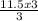 = 11.5moles of oxygen gas
= 11.5moles of oxygen gas