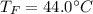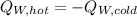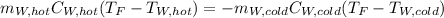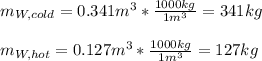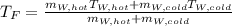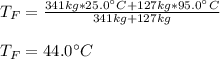Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1

You know the right answer?
If you have 0.341 m3 of water at 25.0 ∘C in an insulated container and add 0.127 m3 of water at 95.0...
Questions


Mathematics, 04.01.2020 04:31




Mathematics, 04.01.2020 04:31

Geography, 04.01.2020 04:31


Mathematics, 04.01.2020 04:31

Mathematics, 04.01.2020 04:31

Biology, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31

Computers and Technology, 04.01.2020 04:31

Social Studies, 04.01.2020 04:31


English, 04.01.2020 04:31


English, 04.01.2020 04:31


Physics, 04.01.2020 04:31