
Chemistry, 20.11.2020 01:00 shelley3135
Using Models to Answer Questions About Systems
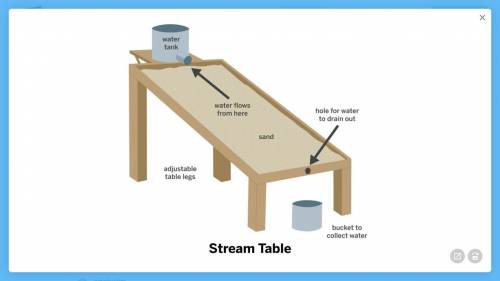
Armando’s class was looking at images of rivers formed by flowing water. Most of the rivers were wide and shallow, but one river was narrow and deep.
Armando’s class thinks that this river is narrow and deep because:
the hill that the water flowed down was very steep, or
the sand grains that the water flowed through were very small.
Armando designed the model below to try to answer the question: Why is this river so narrow and deep?
1a. What features of this model will help Armando answer the question?
1b. How could Armando use this model to test the idea that very steep hills lead to narrow, deep rivers?
1c. How could Armando use this model to test the idea that very small sand grains lead to narrow, deep rivers?
2. Armando thinks that it is the very steep hill that makes this river narrow and deep, but his classmate thinks it is the small size of the sand grains that the water flows through. What results from the model would be evidence that Armando’s idea is correct?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3


Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
Using Models to Answer Questions About Systems
Armando’s class was looking at images of rivers form...
Questions

History, 02.10.2019 23:30


History, 02.10.2019 23:30


Biology, 02.10.2019 23:30


Biology, 02.10.2019 23:30


Mathematics, 02.10.2019 23:30


English, 02.10.2019 23:30

Mathematics, 02.10.2019 23:30

History, 02.10.2019 23:30

Biology, 02.10.2019 23:30

English, 02.10.2019 23:30


Health, 02.10.2019 23:30


Social Studies, 02.10.2019 23:30



