
Chemistry, 19.11.2020 19:00 makaylaf9479
The equilibrium constant Kc for the reaction below is 0.00584 at a certain temperature. Br2(g) ⇌ 2Br(g) If the initial concentrations are [Br2] = 0.0345 M and [Br] = 0.0416 M, calculate the concentrations of these species at equilibrium.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
The equilibrium constant Kc for the reaction below is 0.00584 at a certain temperature. Br2(g) ⇌ 2Br...
Questions

Health, 23.08.2019 20:30



Mathematics, 23.08.2019 20:30

Mathematics, 23.08.2019 20:30


Chemistry, 23.08.2019 20:30


Physics, 23.08.2019 20:30




English, 23.08.2019 20:30

Chemistry, 23.08.2019 20:30

Biology, 23.08.2019 20:30

History, 23.08.2019 20:30

Mathematics, 23.08.2019 20:30

English, 23.08.2019 20:30


Biology, 23.08.2019 20:30

![Q_C = \dfrac{[Br]^2}{[Br_2]} = \dfrac{(0.0416)^2}{(0.0345)}= 0.05016](/tpl/images/0913/4933/a957d.png)
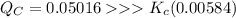
![K_c = \dfrac{[Br]^2}{[Br_2]} = \dfrac{(0.0416-2x)^2}{(0.0345+x)} = 0.00584](/tpl/images/0913/4933/fba5c.png)


