Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fe...

Chemistry, 17.10.2019 03:30 amberchule9681
Iron(ii) sulfide reacts with hydrochloric acid according to the reaction:
fes(s)+2hcl(aq)→fecl2(s)+h2s(g)
a reaction mixture initially contains 0.240 mol fes and 0.646 mol hcl.
what amount (in moles) of the excess reactant is left?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
You know the right answer?
Questions

Mathematics, 14.11.2019 03:31

History, 14.11.2019 03:31

Mathematics, 14.11.2019 03:31

History, 14.11.2019 03:31


Biology, 14.11.2019 03:31


Biology, 14.11.2019 03:31

Mathematics, 14.11.2019 03:31







Mathematics, 14.11.2019 03:31




Mathematics, 14.11.2019 03:31

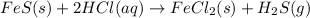
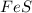 react with 2 mole of
react with 2 mole of 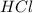
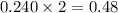 moles of
moles of 

