I’ve tried and tried to do this but now I’m stuck.
Using the Virtual Lab
You have a summer in...

Chemistry, 19.11.2020 01:00 mrsqueenbabe516
I’ve tried and tried to do this but now I’m stuck.
Using the Virtual Lab
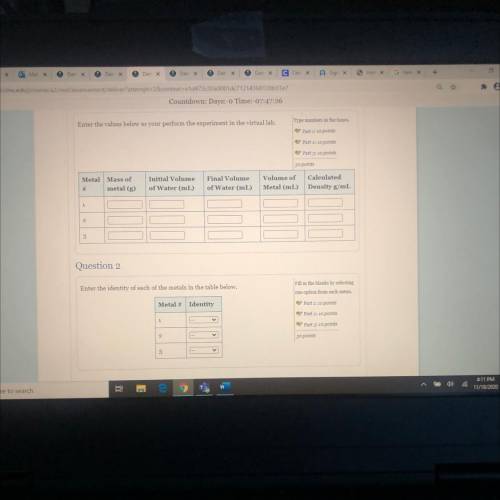
You have a summer internship working in a jewelery laboratory where your job is to explore the properties of
some alloys of silver, rhodium, titanium and platinum in order to make brighter jewelry at a lower cost.
One day while preparing an alloy, you accidentally left three bottles near the melting furnace and the labels
became charred and unreadable. OH NO! You now have three unlabeled bottles of metals. Your task perform
experiments to identify the metals in each of the bottles.
From a chemical handbook available in your lab, you find the following densities:
Metal
Density (g/cm3)
titanium 4.506
silver
10.5
rhodium
12.4
platinum 21.45

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2


Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Questions


History, 13.04.2020 18:58



Computers and Technology, 13.04.2020 18:58


Geography, 13.04.2020 18:58




Social Studies, 13.04.2020 18:59




Health, 13.04.2020 18:59

Mathematics, 13.04.2020 18:59

Health, 13.04.2020 18:59


History, 13.04.2020 18:59



