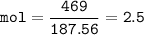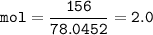Chemistry, 18.11.2020 21:10 MickeyAppleX
In this stoichiometry problem, determine the limiting reactant: Aqueous copper(II) nitrate reacts with aqueous sodium sulfide to produce aqueous sodium nitrate and copper(II) sulfide as a precipitate. In this reaction 469 grams of copper(II) nitrate were combined with 156 grams of sodium sulfide to produce 272 grams of sodium nitrate. Select all that apply.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2



You know the right answer?
In this stoichiometry problem, determine the limiting reactant:
Aqueous copper(II) nitrate reacts w...
Questions

SAT, 09.11.2020 21:00

Mathematics, 09.11.2020 21:00


Mathematics, 09.11.2020 21:00

Law, 09.11.2020 21:00

Spanish, 09.11.2020 21:00



Mathematics, 09.11.2020 21:00

Mathematics, 09.11.2020 21:00



Mathematics, 09.11.2020 21:00

Social Studies, 09.11.2020 21:00

Physics, 09.11.2020 21:00


Business, 09.11.2020 21:00

Mathematics, 09.11.2020 21:00

Mathematics, 09.11.2020 21:00