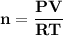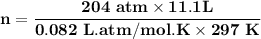Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
A steel cylinder for scuba diving contains 11.1 L of compressed air. The pressure inside the cylinde...
Questions


English, 07.01.2020 19:31



Mathematics, 07.01.2020 19:31



Business, 07.01.2020 19:31


History, 07.01.2020 19:31

Biology, 07.01.2020 19:31

Advanced Placement (AP), 07.01.2020 19:31

Mathematics, 07.01.2020 19:31



Mathematics, 07.01.2020 19:31

Business, 07.01.2020 19:31


Mathematics, 07.01.2020 19:31